Scientists have 3D printed a small heart, complete with blood vessels and chambers. Published this past April, the organ was printed using cardiac cell “bioink” from human iPSCs. Then a few weeks later, researchers printed a “lung” that delivers oxygen to a meticulously entangled vascular network. According to David Thompson, senior R&D manager, in vitro safety systems, at MilliporeSigma, the 3D cell culture space is rapidly expanding. “This includes not only the types of cells being used, such as primary cells, iPSCs, and immortalized cell lines, but also co-culture models that include support cells, for more complex, functional tissue development.”
3D culture runs the gamut from spontaneously forming spheroids to complex bioprinted organs. And as newer methods reduce some of the headache that comes with 3D culture, it’s becoming more mainstream and less the stuff of sci-fi fantasy. Models are becoming more intricate, precise, and being generated faster (sometimes within hours).
Jan Lichtenberg, CEO and co-founder of In Sphero, says that cells grown in 3D look and behave more like cells from human patients. In addition to metabolic disease and cancer, their 3D models are used for preclinical drug safety and efficacy. “These models typically represent the smallest functional unit of a tissue, so that scientists can minimize confounding factors when setting up experiments to assess cellular responses to drug treatments.”
Spheroids’ sphere of influence
Scientists are used to how cells behave in two-dimensions, but that extra dimension can sometimes throw them for a loop. It isn’t always clear if cell lines or primary cells will work well in a spheroid suspension culture. “Cells act very differently, depending on the tissue type, species, health of the donor, and even the process by which the cells were isolated or derived,” says Thompson. He suggests testing different culture conditions for each cell type of interest and notes that “spheroid qualified” cells are now on the market taking the mystery out of whether or not cells will form spheroids and what protocol and reagents to use.
Hilary Sherman, senior applications scientist at Corning Life Sciences says, “Scaffold-free 3D cultures can be much easier and more straight forward to work with compared to 3D models that require a scaffold. Additionally, they tend to be more scalable and amenable to high-throughput applications due to compatibility with liquid handling and instrumentation for analysis.”
Although scaffold-free spontaneously forming spheroids (or microtissues) are straightforward in theory, the process requires enormous attention to detail. “Almost any talented graduate student or postdoc can produce a plate of spheroid models using a tumor cell line. But for pharmaceutical applications, you need a source for standardized models that are uniform in size and functionally robust, otherwise your results may not be reliable or reproducible,” explains Lichtenberg.
His company engineers scaffoldless co-culture models that consist of two or more cell types and immune components. The 3D Select™ process removes necrotic or unhealthy cells from cultures just prior to producing spheroids, with a proprietary hang-drop method or standing drop plates. “They mimic human physiology more closely and survive in culture for several weeks, so that it is possible to conduct longer-term studies with multiple drug dosings, similar to treatment regimes patients would receive at the clinic.” The company has successfully used primary cells, tumor cell lines, and iPSCs in their models and offers several 3D InSight™ platforms that include everything needed for experiments, including media, inducers, and plates. “[These models] offer the best balance of biological complexity and technical simplicity,” notes Lichtenberg.
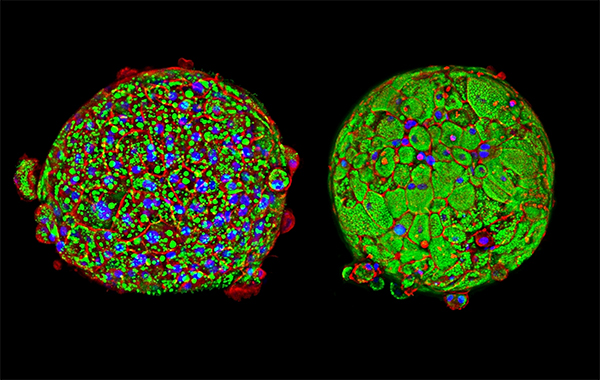
Image: This InSphero 3D InSight™ Liver Disease Model shows tight tissue formation and changes in morphology under healthy control conditions (left) and diabetic conditions (right) induced with a specially formulated media that contains high glucose levels and free fatty acids, mimicking dietary conditions that can cause NAFLD.
Luring cells together ... with magnets
When cells don’t want to clump together (or won’t do so quickly enough), they can be pushed together with magnetic force. In the lab of Ishwar Puri, professor of mechanical engineering and biomedical engineering at McMaster University, breast cancer cells can be magnetically “printed” into 3D spheres in six hours. The lab magnetizes cell media by adding a magnetic salt hydrate, such as gadopentaic acid (Gd-DTPA), an FDA-approved MRI contrast agent. ”When a magnetic field is applied, Gd-DTPA moves toward the magnets, displacing the cells to a predetermined region of minimum magnetic field strength. This seeds the formation of a 3D cell cluster,” explains Sarah Mishriki, a Ph.D. candidate in the School of Biomedical Engineering and member of the Puri lab. They have successfully used this technique on a variety of cell types, including cancer, endothelial, and stromal lines.

Image: Rearranging the topology of the external magnetic field causes cells to form different geometries. As shown, an alternating N-S-N-S magnet arrangement produces a minima in the magnetic field gradient at the magnet intersection, displacing cells towards the center. 3D structure on ULA surface (top) and attached to proliferating 2D culture on treated surface (bottom). Image courtesy of McMaster University.
Mishriki says that using magnets accelerates reproducible production of 3D structures without inserting scaffolds. Magnets arranged in different orientations outside of the culture plate generate distinct magnetic zones that can displace cells into different geometries, 3D as well as intermediates between 2D and 3D. And by using multiple cell lines in the assembly process, “the method can be used to model physiologically relevant cell landscapes, mimicking the unique hierarchy and microarchitecture of cells and tissues found in the body.”
Greiner Bio-One utilizes magnetism in a different way; instead of magnetizing media, their NanoShuttle™-PL 3D magnetizes cells. “We use a magnetic nanoparticle assembly of gold and iron oxide nanoparticles functionalized with Poly-l-lysine that allows the nanoparticle to stick to the cell membrane electrostatically,” explains Glauco Souza, director of global business development & innovation at Greiner Bio-One.

Souza notes that the nanoparticles have been used with numerous cell types in many publications. Depending on the cell type and model, scientists may choose to utilize magnetic levitation or bioprinting for generation of spheroids. A comparison can be found here. “Promoting cell-cell interaction or pushing cells together can generate more robust 3D cultures. This seems especially true when handling cells directly from in vivo tissue,” he says.
Scientists might be most attracted to the system’s ease of use, manipulation, and scalability. It uses flat surfaces, needed when performing high-resolution microscopy or high-content screening.
Image: Greiner's 96 well Spheroid bioprinting kit.
Souza says that adding the magnetic component to 3D culture allows scientists to maintain a 2D-like workflow. A good thing, since relatively straightforward tasks, like changing media, can be cumbersome with 3D culture. Instead, magnetic forces can be used to hold spheroids to the plate during aspiration. The MagPen™ is another option, allowing spheroids to be easily picked up and transferred between vessels. “We use the magnetic field as a surrogate for cells attaching to plastic,” notes Souza.
Building better models with bioprinting
Advances in scaffold materials, polymeric biomaterials used to support cells in 3D, allows for improved tissue architecture, says Thompson. Especially when used in conjunction with bioprinting.
He explains that scaffold materials range from decellularized animal tissues, in which tissue is stripped of its cells leaving behind only the extracellular matrix, to polymerized synthetic or natural proteins. “Researchers are now 3D printing with these biomaterials to form a tissue model layer-by-layer that resembles the architecture seen in the human body.”
Bioprinting requires bioinks. “Bioinks are hydrogels mixed with cells that can be printed to create and support cell-based 3D structures. They are made from a variety of materials including but not limited to collagen, Matrigel, alginate, laminin, and gelatin to name a few,” explains Sherman. These are then extruded through a nozzle in liquid form and polymerized by light or temperature.
Thompson says that there is a need for improved bioinks with better physiological properties. “One promising area of research is the development of patient-specific bioinks that incorporate autologous/patient-specific biological factors for tissue engineering applications.”
Although there are hurdles to working with more complex and costly cultures, it’s clear that 3D cultures are already revolutionizing biomedical research. Says Lichtenberg, “In the 10 years since we started working with 3D cell culture, we’ve seen 3D tissue models evolve from a novelty to a must-have tool that reliably mimics human responses to drug dosing. We’re looking forward to continuing to provide innovative solutions that will ultimately lead to new cures and benefit human health.”