For years, drug discovery took a “target-first” approach with scientists hunting for potential medicinal compounds that could bind and influence an implicated gene or molecule. The hope was that target modulators would translate to the therapeutic realm and give rise to new treatments.
Roger Clark, group leader of high-throughput screening biology and head of compound management at Charles River, says that the human genome project and the drive to measure success against early milestones in drug discovery projects spurred on this reductionist approach. But phenotypic drug discovery (PDD) has fallen into favor again as scientists realize that despite decrypting pathways and identifying specific target modulators from screening vast compound libraries, success of purely target-based approaches is not as high as originally predicted.
Clark explains that, while successful in many cases, focusing only on targeted approaches has yielded late-stage failures across the industry. “Lack of in vivo efficacy, failure to deliver clinical endpoints, or unpredicted tox effects has refocused many organizations to look again at earlier relevant phenotypic data and to balance their portfolios accordingly across target-based and PDD programs.”
When researchers don’t have any identifiable molecules associated with the disease model (or cannot isolate it for in vitro assays) PDD might also prove more useful in the drug search. Without a target in mind, the focus is on screening for compounds that cause an observable change in the disease model, instead of honing in on a very specific molecule or signaling pathway. Hilary Sherman, applications scientist at Corning, notes that this approach can be limiting. “Targeted approaches require a more in-depth understanding of the disease mechanisms, which is not always well understood.”
PDD is a kind of target-agnostic approach, says Jacob Tesdorpf, senior director imaging and detection at Perkin Elmer. “Adoption of a PDD strategy would imply that the researcher has very little prior knowledge of (or at least little bias toward) the mechanisms of action of potential drug candidates.” He adds that the details of how the drug works are secondary to its phenotypic effect on the model system. “PDD therefore increases the chances to find untapped mechanisms of action and first-in-class drugs.”
Shushant Jain, group leader biology at CRL, notes, “In almost all complex multifactorial diseases, where the underlying pathogenic mechanism is unclear or is due to perturbation of multiple pathways, PDD is proving itself useful as it is able to visualize multiple phenotypes or pathways simultaneously.”
PDD’s resurgence is fueled by the rise of more high-quality, disease-reflective animal and cell-based models. “New and improved tools for cell-based phenotypic screening and a lower threshold for biological understanding have helped to increase PDD’s popularity,” says Sherman. “Sometimes the highly specific models from targeted approaches might miss something, or could be based on faulty assumptions.”
Blake Anson, senior director of marketing and strategic alliances at Stemonix, explains that in diseases of multiple mechanisms using a single targeted approach can be cumbersome. “[In the field of] neuronal disorders, new in vitro models are providing a rich and relevant landscape that can be mined at the holistic rather than molecular level.”
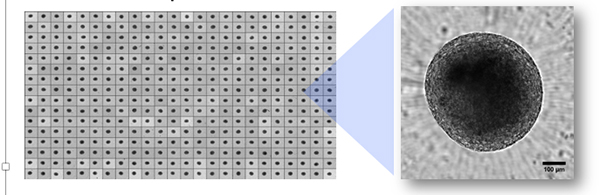
Image: microBrain®3D spheroids are supplied ready-to-use in 96 or 384 well plates with a single spheroid per well as shown above. Composite image of a 384 well plate ready for shipment and assay. High-resolution spheroid imaging performed with the ImageXpress Micro Confocal high-content microscope and stitched together using MetaXpress software (Molecular Devices). Image courtesy of Stemonix
Powerful imaging and predictive assessments
“A high-content approach to phenotypic screening provides multi-parametric data that can be incorporated into a nuanced assessment of, for example, the overall effect of a compound on a system,” says Tesdorpf. High-content screening (HCS) is compatible with newer cell models such as primary, iPSC-derived, co-culture, and 3D, and can offer detailed phenotype measurements at relevant throughput. “While a decade ago most HCS assays relied on established cell lines cultivated in two dimensions, today an increasing number of assays use more complex systems for greater physiological relevance and better representation of in vivo environments.”

Image: HeLa cells stained with Hoechst 33342 (nuclei) and TRITC-Phalloidin (actin cytoskeleton), imaged using the Opera Phenix™ high-content screening system and analyzed using Harmony® software.
Blake says that these innovative models should be supplied at industrial levels through quality-controlled protocols. To that end, Stemonix provides both 3D and 2D human neuron/astrocyte co-cultures, as well as human cardiac cells on a structured microHeart plate, which allows for the passive formation of native-like cardiac fibers. Both are iPSC-derived and come assay ready in multiwell formats.
Corning’s specialized surfaces and cultureware also help to mimic the in vivo environment. Sherman highlighted Matrigel® matrix, Corning spheroid microplates, and Transwell® permeable supports and noted that many products are well-suited for imaging assays, such as Corning high-content microplates and the new Elplasia® plates.
Aside from reliable models, Blake thinks high-content imaging is one of the more successful approaches to PDD and notes that it can simultaneously monitor different processes and cellular structures. And Tesdorpf notes that Cell Painting, which uses a set of dyes to stain a variety of cell structures along with image analysis, can be used to create detailed phenotypic fingerprints. These fingerprints can be analyzed at the single-cell level, important since taking the average of cells in a well can sometimes mask a significant phenotypic response.
Perkin Elmer specializes in image-based phenotypic screening and Tesdorpf underscored the HCS portfolio, which includes either the Opera Phenix® or Operetta® CLS™ high-content imaging systems, and Harmony® imaging and analysis software, which uses a workflow-based interface. “[These] systems can be utilized with models ranging from simple 2D cell cultures, to iPSC-derived co-cultures, 3D organoids, or small in vivo models such as zebrafish larvae.” Harmony software can generate phenotypic fingerprints, quantify subtle changes, and be trained to recognize relevant phenotypes with machine learning.
“Multiple approaches are being used for PDD, mainly fueled by improvements in ‘omic’ technologies and the associated automation/miniaturization to make those approaches cost effective at a reasonable scale,” says Jain. Various technology platforms or screening approaches lend themselves well to PDD at an HTS scale including: high-content imaging, high-throughput flow cytometry, and next-generation sequencing (NGS).
At CRL, the recent collaboration with AstraZeneca means that now clients have access to Charles River's screen expertise alongside certain HTS technologies available within AstraZeneca labs, further enhancing the portfolio of bespoke assay options already available. Clark notes that Charles River clients are able to screen either the in-house compound library or their own in the PDD assays offered by Charles River.
Target deconvolution
Aside from using a high-quality, relevant model, which is perhaps the biggest predictor in the success of PDD, target deconvolution remains one of the most sizeable challenges. Jain notes that any hit obtained through PDD screening will require a fair amount of work to identify the targets and pathways involved. And even after they are known, further effort will be needed to understand their relevance in the disease setting.
Clark says that although hit validation and target deconvolution are issues, there are now several promising methods available. “They include genomic assays with the use of CRISPR, transcriptome analysis, machine learning to cluster hits around references compounds with known mechanism of action, and CETSA MS [cellular thermal shift assays with mass spectrometry] that allows detection of target engagement in live cells for thousands of proteins at the same time.” CRL also employs a technology platform called Capture Compound Mass Spectrometry (CCMS), which uses UV reactive probe molecules built around chemistry identified from a PDD screen to capture and pull down protein targets. Those targets captured and identified via mass spectrometry can be used to bulid a shortlist of targets and pathways implicated in the phenotype of interest.

Although more time and effort may be required up front with PDD, the molecules that do get shortlisted already have the ability to modulate disease phenotype. “The unbiased approach is leading to new medicines and opening new potential therapeutic pathways,” Anson adds. “While yearly numbers can fluctuate, the industry is at a point where the majority of new first in class drugs were discovered via phenotypic screens.”
Image: microBrain®3D spheroids are composed of neurons and astrocytes as shown by immunolabelling. Astrocytes labelled with glial fibrillary acidic protein (GFAP; red), neurons labelled with microtubule-associated protein 2 (MAP2; green), nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Antibody labelling was performed with primary antibodies directed against the cellular epitope and fluorophore conjugated secondary antibodies for visualization. Antibodies were purchased from Millipore (MAP2) and Abcam (GFAP), DAPI was purchased from ThermoFisher Scientific and images were taken on the ImageXpress Micro Confocal high-content microscope (Molecular Devices). Spheroid is ~600mM in diameter. Image courtesy of Stemonix.