Imaging flow cytometry (IFC) is a technique that combines conventional flow cytometry with microscopy to both analyze and image extremely large numbers of individual cells. In recent years, it has become increasingly popular as researchers strive to gain deeper insights from limited sample material at the single-cell level. Here, we provide a general overview of imaging flow cytometry and explain how machine learning and artificial intelligence (AI) are helping to make it more accessible.
Not a new technique
Imaging flow cytometry has been around for decades, with attempts to integrate video imaging with flow cytometry being described as early as 1979. According to Tina Silovic, Ph.D., Application Scientist at CytoBuoy, the adaptation of confocal microscopy to flow cytometry in the 1980s represented an important breakthrough for the technique, and has paved the way to imaging flow cytometry as we know it today. “Technical problems that have had to be overcome during the development of IFC include triggering imaging in flow, positioning particles in the plane of focus, freezing the motion of rapidly moving objects, and identifying suitable light sources for strong, pulsed illumination,” she explains. “Until fairly recently, storing large numbers of images quickly also presented a major challenge.” As these issues have been addressed, more researchers have adopted the technique.
Spatial resolution provides more information
Unlike conventional flow cytometry, where recorded signals are generated by whole cells, IFC offers spatial resolution. Brian Hall, Product Manager for Amnis® Imaging Flow Cytometry at Luminex, comments that this is important because it provides orders of magnitude more information about the sample being evaluated. “Knowing not only the intensity of a probe but where that probe is located allows researchers to better understand their current cytometry applications and ask totally new types of questions,” he says. “By providing an image of each cell, IFC enables researchers to see where proteins reside, and to answer critical questions like are they colocalized with other proteins, are they expressed uniformly or in puncta, are they on the cell membrane, and is that membrane changing shape? Each of these morphological observations can be crucial to understanding the biology of the cell.”
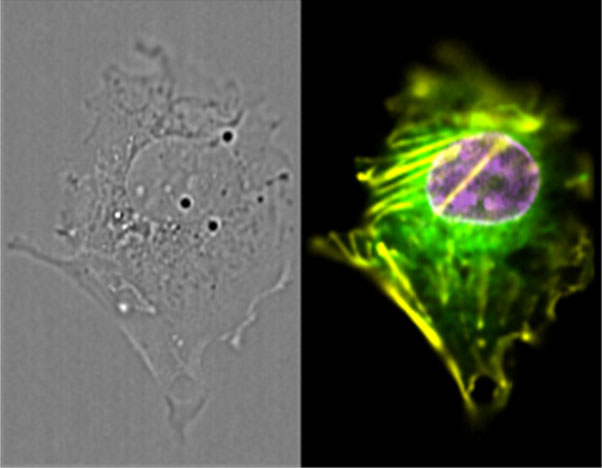
Image: U2OS cell fixed in culture, with the brightfield and three fluorescent colors imaged simultaneously in flow using an Amnis® ImageStream®X Mk II with a 40x objective. The cell was genetically modified using zinc finger nucleases to add red fluorescent protein (RFP) to Actin B, green fluorescent protein (GFP) to Tubulin a1B, and blue fluorescent protein (BFP) to the Lamin B1 nuclear envelope protein. Image provided by Luminex.
Supporting diverse applications
Imaging flow cytometry has been applied to countless research fields, largely championed by researchers wishing to introduce the advantages of imaging into cytometry applications, and the power of statistics to microscopy. Hall reports that applications published recently citing use of the Amnis® ImageStream® (one of Luminex’ IFC platforms) include measuring target cell death in CRISPR/CAS9 modified CAR-T cells, binding of antigen-presenting cells and formation of the immune synapse, LC3 clustering in autophagic cells, nuclear localization of DNA transcription factors, cyclin dependent cell cycle progression, measuring the apoptotic index after drug treatment, colocalization of proteins, and uptake of extracellular vesicles. Performing such experiments using conventional flow cytometry or microscopy could mean valuable insights being missed due to the inherent limitations of these techniques.
Browse Imaging Cytometers Search Now Search our directory to discover imaging flow cytometers for your research.
IFC has also advanced the field of aquatic microbiology, where it has especially been useful for in situ observation. “Monitoring the dynamics of different plankton species forms the basis for an ecological status assessment of water bodies,” notes Silovic. “By using IFC to analyze different ecological/physical compartments simultaneously (e.g., bacteria, phytoplankton, small zooplankton, parasites, eggs, fungi, solitary cells, colonies, aggregates, filaments etc.), researchers can monitor any sudden or unexpected changes that may be indicative of anomalous conditions such as harmful algal blooms. Our CytoSense imaging flow cytometer can be made submersible or transformed into a moored, autonomous buoy to support these types of applications, and is used worldwide for studying spatio-temporal variability in plankton communities.”
“A study published in May of this year highlights the advantages of IFC very well,” says Hall. “In the publication, the authors describe how IFC was successfully used as an alternative to flow cytometry or microscopy for performing a micronucleus (MN) assay—a broadly used method for identifying cytogenetic damage in cells caused by various toxins. During traditional flow cytometry, the fluorescent cells are lysed, and the MN are counted. However, some cells may have multiple MN, and debris absorbing the DNA dye can give rise to false positives. The microscopy approach overcomes these problems, but is labor intensive and requires expertise to ensure true MN are identified; it also lacks statistical robustness since typically only one thousand cells are scored. Using IFC, the researchers were able to more effectively remove debris and, by looking at intact cells, could count the number of MN per cell. Furthermore, collecting many thousands of images allowed for improved statistics and the use of AI, permitting effective automated scoring.”
Becoming more accessible
Some of the main barriers to adoption of IFC have been centered around the challenges of learning how to effectively use the systems and analyze the data. Hall mentions that as the technology has become more commonplace, the publication of additional methods and protocols has aided experimental design and, in parallel, IFC instrumentation has become faster and easier to use. Yet, there is still a steep learning curve on the analysis side.
“Many researchers find the task of fully understanding hundreds of morphology-based features and how best to use them a bit daunting,” he says. “To address this problem, new software tools are designed to make IFC analysis available to all levels of user. These include wizard-based analysis tools for those who are new to the technique, and more advanced machine learning tools that can be adapted to quantify almost any morphology. These are complemented by artificial intelligence software (such as Amnis® AI) that uses deep learning to create a model of the sample and automatically identify each cell type based on its fluorescent patterns and morphology. Advantages of AI are that it maintains the expertise of individual researchers after they leave the lab and it doesn’t require an extensive amount of knowledge in morphology-based classifiers.”
Silovic comments that, at present, dataset size and collection speed are outpacing the development of automatic methods of recognition and classification, at least within the field of aquatic microbiology. “Machine learning algorithms still depend heavily on manual annotation,” she says. “In my personal point of view, this bottleneck would ideally be solved by pooling the expertise of taxonomists globally to create an open-source phytoplankton database of images, identified to the species level. This would allow machine learning techniques to be used to train computers to do the same thing. Until then, efforts are pretty much scattered around particular instrumentation or research groups, but with global initiatives focused on making this happen, we will hopefully see the outcomes soon.”