Abstract
Many innovations in materials chemistry have been developed to improve the performance of photovoltaic and light-emitting devices. Although the major target of these advances is not biological, we have adapted a number of materials advances to improve the performance of biological fluorescence assays. Quantum dots and light-harvesting polymer materials are very efficient absorbers, which improves their brightness in fluorescence imaging and detection by order(s) of magnitude relative to conventional fluorescent probes. Appropriate chemical modification of the materials can produce high-performance biological detection reagents that improve the sensitivity of assays and enhance the detection limit in fluorescence microscopy.
Introduction
Fluorescence detection has become a mainstay of basic biological investigation and drug discovery [1], and recently has even crossed over into pre-clinical validation using animal models [2]. Many of these approaches are based on fluorescent probes derived from a relatively small set of molecular scaffolds developed over the past fifty years. Analogs of fluorescein, rhodamine and indocyanine dyes are the dominant probes, and have a set of properties that are largely limited by these scaffolds (Figure 1). Systematic chemical modification of these basic scaffolds have resulted in substantial improvements in biological nonspecific binding and fluorescent stability under the microscope, but the overall chromophore brightness is set largely based on the properties of the scaffold. For bright and sensitive detection, single fluorescent molecules are simply not bright enough for most biological experiments in high-throughput microscopy contexts.
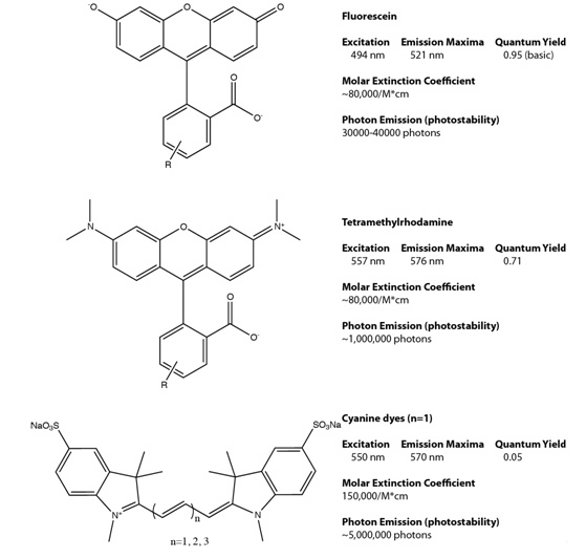
Figure 1- Common dye scaffolds used for biological detection. Fluorescein, rhodamine and cyanine dye scaffolds define the general properties of the family of fluorescent probes that are based on this molecular framework.
Much of our work has focused on providing order-of-magnitude improvements in the brightness and photostability with fluorescent probes that can be used for biological microscopy. In these efforts, we have frequently found solutions developed in Materials Science disciplines that can be adapted for high-performance biological detection. This cross-fertilization has more frequently proceeded in the other direction, namely using biologically inspired or derived approaches for materials science, an example is the work of Angela Belcher, who has integrated biological assembly with inorganic materials surfaces for a variety of device integration and patterning applications [3,4]. Due to the general complexity involved in adapting non-aqueous materials for biological compatibility, it has been more complex to adapt materials for biological application. Clearly, this is changing, as integrated biosensor and microfluidic devices are now available that can process samples and cells [5], some of which even power next-generation sequencing systems [6].
Fluorescence Detection with Quantum Dots
In contrast to the basic structures highlighted in Figure 1, quantum dots have unique properties. Quantum dots are nanometer-sized pieces of crystalline semiconductor materials, such as CdSe and Si, which display strongly size-dependent spectra. For example, a 2 nm CdSe particle is a green emitter at 515 nm while a 6 nm CdSe particle is a red emitter at 630 nm. In addition to this attractive size-dependent emission property, the quantum dots display unusual absorbance spectra, because they are based on semiconductor materials. The absorbance of the quantum dots increases as the distance from the emission band is increased, owing to the “band structure” of the underlying semiconductor material. This increased absorbance is still effectively coupled to the emission of the quantum dot, resulting in a direct increase in brightness. Figure 2 highlights some of the essential features of a quantum dot material relative to Cy3, a typical dye molecule. The major challenge with the use of quantum dots for biological detection has been the incompatibility of the high-quality quantum dots with biological buffers [7,8]. The highest-quality synthetic routes for quantum dots prepare materials that are highly soluble in organic solvents, and a variety of phase transfer chemistries have been developed to overcome this problem [9-12]. Nevertheless, quantum dots, as prepared for biological experiments, remain relatively large (10—20 nm) compared to synthetic dyes (1—2 nm), and this poses some issues in biological labeling approaches.
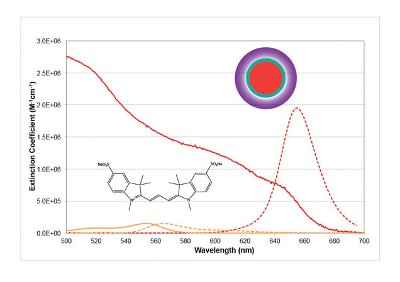
Figure 2- Quantum dots have substantially different spectroscopic properties than conventional fluorescent probes. The molar extinction coefficients of quantum dots are substantially higher than even the best fluorescent dye scaffolds, resulting in substantially higher detectability and sensitivity. The quantum dot structure is an engineered nanomaterial, consisting of several layers, the core (red) defining the spectral properties, the shell (green) defining the quantum yield and the coat (purple) defining the solubility and assay performance (e.g., nonspecific binding, conjugation chemistry).
Quantum dots have become a standard tool for analysis of trajectories of biological receptors on living cells, and have revealed new details related to trafficking of EGFR and other important therapeutic targets [13,14]. While these experiments were carried out in a sensitive microscope, at a single molecule level, other approaches utilizing the quantum dots as a cell-line tag have allowed multiplexing cellular response assays in a single well [15,16] under high-throughput conditions.
These materials have also been used in a range of clean biophysical experiments that may give rise to some important screening applications. Recent work has demonstrated that one or many distinct proteins can be labeled with quantum dots and then assayed as they interact with stretched DNA, revealing transcriptional dynamics [17], nuclease reactivity [18] or DNA repair processes [19]. While these have started as biophysical experiments, the methodologies used are surprisingly straightforward, and could provide a powerful and quantitative approach to assaying these molecules in drug discovery assays.
Innovations in quantum dot chemistry have provided a range of distinct surface modifications and coupling chemistries to adapt these materials to routine biological applications [7,8,10-12].
Genetically Targeted and Activated Light Harvesting Dendrons
Intrinsic fluorescent proteins have significant advantages relative to exogenous probes like dye molecules and quantum dots. Labeling of a protein is accomplished easily at a genetic level using conventional molecular biology tools. The fluorescent protein-labeled target (if the construct is biologically functional) carries its fluorescent label wherever it goes, allowing simple microscopy to reveal protein behavior in living cells. Fluorescent proteins are available in a wide range of colors and environmentally sensitive formats based on fifteen years of consistent development [20]. To substantially improve the brightness of genetically targeted reporters, a radical innovation is needed. The chromophore in fluorescent proteins is derived from its primary amino-acid sequence, and typically has a high fluorescent quantum yield and a modest extinction. Synthesized fluorescent dyes can have higher extinction, resulting in two-fold brightness improvements. But how can one achieve a 10-100-fold enhancement in brightness at a single molecule level? The answer lies in the field of light-harvesting polymers (LHPs).

LHPs have been prepared for a range of devices, including photovoltaics and light-emitting diodes. The absorbance of LHPs is enhanced using non-radiative energy transfer from an antenna array of donor molecules to a single acceptor moiety [21]. This multiplies the absorbance of the structure, but also provides emission from a single localized emitter. These materials have generally been prepared with biologically incompatible dyes in biologically incompatible solvents. Attempts to use multichromophore structures for biological detection have suffered from self-quenching interactions among the dye molecules and increased nonspecific binding to off-target biological structures. Our results have shown that the key to using these materials effectively for cellular labeling lies in both targeting and activating the fluorescent structure at the protein of interest (Figure 3). Because unbound probes are essentially nonfluorescent, off-target binding generates no background [22,23]. Using first-generation light harvesting polymers (also called dyedrons) with 4 donor molecules, we were able to obtain 7-fold improved sensitivity in microscopy- and flow cytometry-based experiments. Continued improvements to the LHP constructs may deliver substantial improvements beyond this initial level.
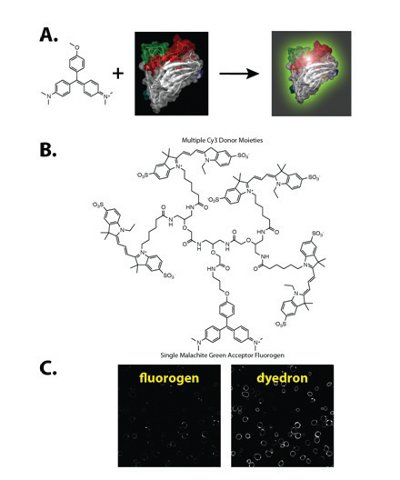
Figure 3- Fluorogen Activating Peptides and Light Harvesting Polymer (dyedrons) for detection. A. The fluorogen activating peptide binds to a nonfluorescent dye and activates the fluorescence significantly. B. Light harvesting polymers (dyedrons) made with multiple donor dyes linked to a fluorogen acceptor are effectively quenched free in solution and activated on binding the fluorogen activating peptide. C. Use of dyedrons substantially enhances the brightness and sensitivity of assays, as illustrated labeling fluorogen activating peptide at the surface of cells.
To achieve this activation, we have selected proteins that bind to otherwise non-fluorescent dyes, activating the fluorescence of the molecules by >10,000-fold [22]. We call these proteins fluorogen activating peptides. This approach has the significant advantage of requiring no separation of the bright probes from the labeled cells, as the background activation of the dye is effectively non-existent. Using cell excluded dyes, this labeling approach can be used to screen for activators of receptor internalization [24]. While these experiments were carried out on stably transduced cells with an overexpressed transgene carrying the fluorogen activating peptide, it is possible that use of these light harvesting polymer structures would enable detection of endogenous levels of these receptors due to the high sensitivity of the material.
Conclusions
Materials chemists have developed a wide range of new materials with interesting electronic and optical properties. These materials have provided an interesting set of new detection materials for biological experiments, and have enhanced the sensitivity and multiplexing capability of many common assays and experiments. At a time when the national investment in biological tools is shrinking while the investment in materials chemistry and energy technology is growing, we can hope to see more innovation that can be applied in both materials and biological contexts.
References
- Lavis, L. D.; Raines, R. T.: Bright ideas for chemical biology. ACS chemical biology 2008, 3, 142-55.
- Wellen, J.; Wang, X.; Wan, H.: Optical Imaging: Exploring Noninvasive Molecular Imaging Biomarkers for Drug Discovery and Development. American Pharmaceutical Review 2009, September/October, 140-146.
- Nam, Y. S.; Magyar, A. P.; Lee, D.; Kim, J. W.; Yun, D. S.; Park, H.; Pollom, T. S., Jr.; Weitz, D. A.; Belcher, A. M.: Biologically templated photocatalytic nanostructures for sustained light-driven water oxidation. Nature nanotechnology 2010, 5, 340-4.
- Nam, Y. S.; Shin, T.; Park, H.; Magyar, A. P.; Choi, K.; Fantner, G.; Nelson, K. A.; Belcher, A. M.: Virus-templated assembly of porphyrins into light-harvesting nanoantennae. Journal of the American Chemical Society 2010, 132, 1462-3.
- Kalisky, T.; Quake, S. R.: Single-cell genomics. Nature methods 2011, 8, 311-4.
- Rothberg, J. M.; Hinz, W.; Rearick, T. M.; Schultz, J.; Mileski, W.; Davey, M.; Leamon, J. H.; Johnson, K.; Milgrew, M. J.; Edwards, M.; Hoon, J.; Simons, J. F.; Marran, D.; Myers, J. W.; Davidson, J. F.; Branting, A.; Nobile, J. R.; Puc, B. P.; Light, D.; Clark, T. A.; Huber, M.; Branciforte, J. T.; Stoner, I. B.; Cawley, S. E.; Lyons, M.; Fu, Y.; Homer, N.; Sedova, M.; Miao, X.; Reed, B.; Sabina, J.; Feierstein, E.; Schorn, M.; Alanjary, M.; Dimalanta, E.; Dressman, D.; Kasinskas, R.; Sokolsky, T.; Fidanza, J. A.; Namsaraev, E.; McKernan, K. J.; Williams, A.; Roth, G. T.; Bustillo, J.: An integrated semiconductor device enabling non-optical genome sequencing. Nature 2011, 475, 348-52.
- Bruchez, M., Jr.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A. P.: Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013-6
- Chan, W. C.; Nie, S.: Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016-8.
- Wu, X.; Liu, H.; Liu, J.; Haley, K. N.; Treadway, J. A.; Larson, J. P.; Ge, N.; Peale, F.; Bruchez, M. P.: Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nature biotechnology 2003, 21, 41-6.
- Howarth, M.; Liu, W.; Puthenveetil, S.; Zheng, Y.; Marshall, L. F.; Schmidt, M. M.; Wittrup, K. D.; Bawendi, M. G.; Ting, A. Y.: Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nature methods 2008, 5, 397-9.
- Kairdolf, B. A.; Mancini, M. C.; Smith, A. M.; Nie, S.: Minimizing nonspecific cellular binding of quantum dots with hydroxyl-derivatized surface coatings. Anal Chem 2008, 80, 3029-34.
- Muro, E.; Pons, T.; Lequeux, N.; Fragola, A.; Sanson, N.; Lenkei, Z.; Dubertret, B.: Small and stable sulfobetaine zwitterionic quantum dots for functional live-cell imaging. Journal of the American Chemical Society 2010, 132, 4556-7.
- Lidke, D. S.; Nagy, P.; Heintzmann, R.; Arndt-Jovin, D. J.; Post, J. N.; Grecco, H. E.; Jares-Erijman, E. A.; Jovin, T. M.: Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nature biotechnology 2004, 22, 198-203.
- Lidke, D. S.; Lidke, K. A.; Rieger, B.; Jovin, T. M.; Arndt-Jovin, D. J.: Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. The Journal of cell biology 2005, 170, 619-26.
- Mattheakis, L. C.; Dias, J. M.; Choi, Y. J.; Gong, J.; Bruchez, M. P.; Liu, J.; Wang, E.: Optical coding of mammalian cells using semiconductor quantum dots. Analytical biochemistry 2004, 327, 200-8.
- Wylie, P. G.: Multiple cell lines using quantum dots. Methods Mol Biol 2007, 374, 113-23.
- Wang, F.; Greene, E. C.: Single-molecule studies of transcription: from one RNA polymerase at a time to the gene expression profile of a cell. Journal of molecular biology 2011, 412, 814-31.
- Visnapuu, M. L.; Fazio, T.; Wind, S.; Greene, E. C.: Parallel arrays of geometric nanowells for assembling curtains of DNA with controlled lateral dispersion. Langmuir : the ACS journal of surfaces and colloids 2008, 24, 11293-9.
- Kad, N. M.; Wang, H.; Kennedy, G. G.; Warshaw, D. M.; Van Houten, B.: Collaborative dynamic DNA scanning by nucleotide excision repair proteins investigated by single- molecule imaging of quantum-dot-labeled proteins. Molecular cell 2010, 37, 702-13.
- Tsien, R. Y.: Constructing and exploiting the fluorescent protein paintbox (Nobel Lecture). Angew Chem Int Ed Engl 2009, 48, 5612-26.
- Balzani, V.; Ceroni, P.; Maestri, M.; Vicinelli, V.: Light-harvesting dendrimers. Curr Opin Chem Biol 2003, 7, 657-65
- Szent-Gyorgyi, C.; Schmidt, B. F.; Creeger, Y.; Fisher, G. W.; Zakel, K. L.; Adler, S.; Fitzpatrick, J. A.; Woolford, C. A.; Yan, Q.; Vasilev, K. V.; Berget, P. B.; Bruchez, M. P.; Jarvik, J. W.; Waggoner, A.: Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nature biotechnology 2008, 26, 235-40.
- Szent-Gyorgyi, C.; Schmidt, B. F.; Fitzpatrick, J. A.; Bruchez, M. P.: Fluorogenic dendrons with multiple donor chromophores as bright genetically targeted and activated probes. Journal of the American Chemical Society 2010, 132, 11103-9.
- Fisher, G. W.; Adler, S. A.; Fuhrman, M. H.; Waggoner, A. S.; Bruchez, M. P.; Jarvik, J. W.: Detection and quantification of beta2AR internalization in living cells using FAP-based biosensor technology. Journal of biomolecular screening 2010, 15, 703-9.
Dr. Bruchez has authored 17 patents and 28 peer-reviewed papers at the interface of materials chemistry and biology. Science recognized the development of biocompatible qdots as one of the Top Ten Innovations of 2003. Dr. Bruchez received the 2006 Rank Prize for Optoelectronics for “realization of quantum dot nanocrystals as biological labels.” Dr. Bruchez received a B.S. in chemistry from MIT, and a Ph.D. in physical chemistry from the University of California at Berkeley. He is an Associate Professor in the Departments of Chemistry and Biological Sciences at Carnegie Mellon University and Associate Director of the Molecular Biosensors and Imaging Center.
This article was printed in the 12/20/2011 issue of International Drug Discovery,6,, 6,. Copyright rests with the publisher.