Immuno-oncology harnesses the body’s own immune system to attack and destroy tumor cells. This means that, unlike established cancer treatments such as radiotherapy and chemotherapy, immuno-oncology provides a targeted therapeutic approach that is less likely to damage healthy tissue. Many recent breakthroughs in immuno-oncology research have been underpinned by advances in flow cytometry. These include the development of checkpoint inhibitors, CAR T cell therapy, and cancer vaccines, highlighting the importance of flow cytometry to help deliver essential therapies to patients. Not only has flow cytometry made cell characterization easier, but it has also evolved to provide a level of spatial resolution that can enhance understanding of cellular processes implicated in cancer.
New sample processing methods prevent cell damage for detection of rare events
Sample types that are typically analyzed during an immuno-oncology research study include blood, lymph, bone marrow, and tissue biopsy material. Since many of these are only available in very small amounts, the development of new methods to process them has been key to maximizing the value of immuno-oncology data. Nitin Kulkarni, Ph.D., field application scientist at MicroMedicine, notes that although density gradient centrifugation is still widely used to isolate and concentrate white blood cells from whole blood, gentler methods may prevent valuable information being lost due to cell damage.
“Techniques that capture relevant cell populations while preserving cellular integrity are vital to support laboratory immuno-oncology research efforts,” he says. “Using the  in microfluidics, it is now possible to extract a complete white blood cell population from whole blood with minimal sample perturbation and better, more consistent removal of red blood cells, platelets, and cell debris compared to existing methods.” Maintaining cell viability provides opportunities to progress cells to further laboratory downstream studies, whereas increasing the number of useful events measured without extending timelines yields deeper insight from precious sample material. By moving away from traditional density gradient-based isolation methods, researchers are better able to analyze the complete white blood cell population and detect the rare events that characterize many cancers.
in microfluidics, it is now possible to extract a complete white blood cell population from whole blood with minimal sample perturbation and better, more consistent removal of red blood cells, platelets, and cell debris compared to existing methods.” Maintaining cell viability provides opportunities to progress cells to further laboratory downstream studies, whereas increasing the number of useful events measured without extending timelines yields deeper insight from precious sample material. By moving away from traditional density gradient-based isolation methods, researchers are better able to analyze the complete white blood cell population and detect the rare events that characterize many cancers.
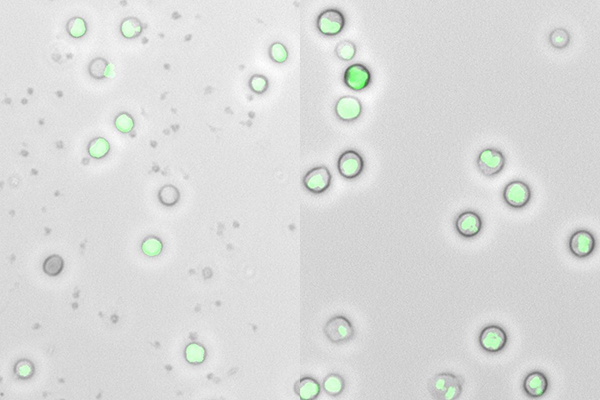
Image: Inertial focusing microfluidics (right) provides better removal of red blood cells, platelets and cell debris than density gradient centrifugation (left). Image provided by MicroMedicine.
Spectral cytometry increases the number of experimental readouts
According to Miguel A. Tam, senior manager, product marketing at BioLegend, another factor driving progress within the field of immuno-oncology research is the development of spectral cytometry instrumentation. “Spectral instruments are equipped with several detectors to capture the entire emission spectra of a fluorophore,” he explains. “So even if two fluors share similar or identical emission peaks, they may still be resolved if there are enough differences in other parts of their emission spectra. In combination with the ever-increasing number of new dyes that are available, this allows researchers to build larger panels and examine a greater number of markers and cell populations in their samples.”
Kulkarni adds that since the new spectral signatures of dyes eliminate the need for compensation, researchers can now measure up to 50 color profiles concurrently by flow cytometry. “Although other technologies such as mass cytometry can also measure a large number of markers effectively, they are expensive and destructive to the cells of interest,” he says. “Flow cytometry is by far the most convenient way to both screen cells using specific markers and still allow for the same cells to be further assessed by FACS to isolate rare cell types for culture or for applications like single-cell sequencing.”
Imaging flow cytometry provides spatial information
"Another exciting development helping to accelerate immuno-oncology research has been the marriage of flow cytometry and microscopy to yield imaging flow cytometry (IFC), reports Haley R. Pugsley, Ph.D. manager and senior scientist at Luminex Corporation. “IFC is fast, sensitive, and quantitative like traditional flow cytometry, but it also provides spatial resolution to locate where in the cell a signal is coming from. This can be extremely useful for immuno-oncology research since it isn’t always enough to know if a cell is positive for a particular protein.”
Pugsley highlights as an example nuclear factor kappa-B (NFκB), a protein that has been shown to play a crucial role in the development of malignant tumors. “In its inactive state, NFκB resides in the cytoplasm, but upon activation it enters the nucleus to bind to target genes,” she says. “IFC is uniquely suited to monitor NFκB activation as it can quantitatively measure this transition.” IFC has also proven valuable in identifying and phenotyping circulating tumor cells, studying cell-cell interactions, and investigating disruptions to cell signaling mechanisms.
Multiplexing is fundamental to CAR T cell characterization
One area where flow cytometry has been pivotal to immuno-oncology research is in supporting the development of CAR T cell therapy. “Without flow, CAR T cell characterization would be incredibly difficult,” explains Tam. “While there are alternative assays to flow cytometry, they are not as scalable to the sheer number of cells that require evaluation in the context of CAR T cell function and overall anticancer immune responses. Flow cytometry provides speed, sensitivity, and, crucially, multiparameter analysis of often very limited sample material.”
“The capability to perform multiparametric analysis at the single-cell level make flow cytometry and IFC particularly well-suited to characterize CAR T cells,” adds Pugsley. “Where IFC has really added to CAR T cell research is in looking at the formation of an immunological synapse. This is the interface between an antigen-presenting cell (in this case a cancer cell) and the CAR T cells, resulting in the killing of the target cancer cell. During an immunological synapse, actin polymerizes at the interface; measuring actin polymerization is therefore a useful way of assessing CAR T cell effectiveness.”
Supporting future discovery
With developments in instrumentation, reagents, and data analysis merging with novel technologies to further extend the utility of flow cytometry to immuno-oncology research, more exciting discoveries are inevitable. Whether these come from studying how the hypoxic tumor microenvironment determines the  of tumor cells, monitoring the role of
of tumor cells, monitoring the role of  in metastasis, or evaluating the vast array of cellular mechanisms that underlie resistance to traditional cancer therapies, only time will tell.
in metastasis, or evaluating the vast array of cellular mechanisms that underlie resistance to traditional cancer therapies, only time will tell.